Safety Profile
Look into the safety data


Ruxolitinib cream was well-tolerated.
Treatment-related TEAEs among patients who applied Ruxolitinib cream at any time were all mild or
moderate (none serious)
Adverse Reactions Associated with Ruxolitinib at 52 Weeks
Adverse Reactions Occurring in Patients Treated with Ruxolitinib through Week 52 In TRuE-V1 and TRuE-V2
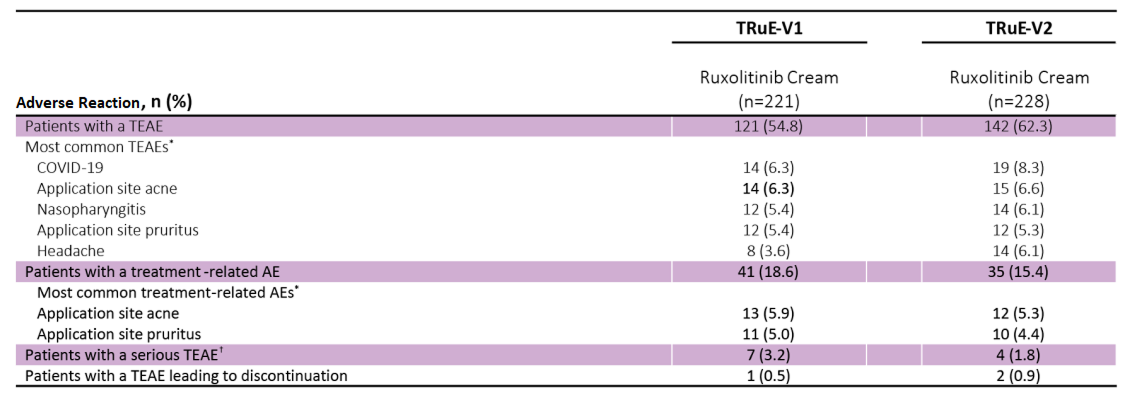
Occurring in ≥2% of patients in any treatment group.
No serious TEAEs were considered by the investigators to be related to treatment.
Reference:
- 10. Rosmarin D, Sebastian M, Amster M, et al. Facial and total vitiligo area scoring index response shift during 104 weeks of Ruxolitinib cream treatment for vitiligo: results from the open-label arm of the TRuE-V long-term extension phase 3 study. Presented at the American Academy of Dermatology Annual Meeting; March 17–21, 2023; New Orleans, LA.